


As a response to the rapidly-growing issue of medical supply shortages across the United States, the Food and Drug Administration (FDA) has authorized the use of certain non-NIOSH-approved disposable filtering facepiece respirators (FFR).
Personal Protective Equipment (PPE), especially N95 masks are tested and certified by the National institute for Occupational Safety and Health, which is a research group as part of the Centers for Disease Control and Prevention (CDC).
In this case, the NIOSH provides safety standards that FFRs must comply with to be considered safe.
Under current circumstances, the FDA believes that these devices will serve a substantial and suitable purpose for protection during this period of shortage caused by the COVID-19 pandemic. Non-NIOSH approved masks are more widely available than those for use and circulation and meet Chinese standards instead of American.
This means that the FDA will authorize the emergency use of authorized respirators for use in healthcare settings by healthcare personnel when used in accordance with CDC recommendations to prevent wearer exposure to pathogenic biological airborne particulates during FFR shortages resulting from the COVID-19 outbreak.
Some things to help indicate the classification of FFRs
Filter Performance - The filter of these masks is evaluated to measure the reduction in concentration of specific aerosols in air that passes through the filter.
Test agent- They aerosol that gets generated during the filter performance test.
Total Inward Leakage (TIL) - The amount of a specific aerosol that enters a tested respirator facepiece through both, filter penetration and face seal leakage. All of this while the wearer performs a series of exercises in the test chamber.
Inward Leakage (IL) - The amount of a specific aerosol entering the tested respirator facepiece, while the wearer breathes normally for 3 minutes in the test chamber.
Pressure Drop - The resistance that air is subjected to as it moves through the medium- such as a respirator filter.
Do you have questions about how to import these FFR masks into the United States?
Simply give us a call at (877) 909-7447 and one of our logisticians will help arrange it for you!

It has been established: ATA Carnets make the import/export experience of non-commercial goods so much easier, however, the usage of this document, has its own complications and concerns. Many Carnet holders often show concern about when to use each part
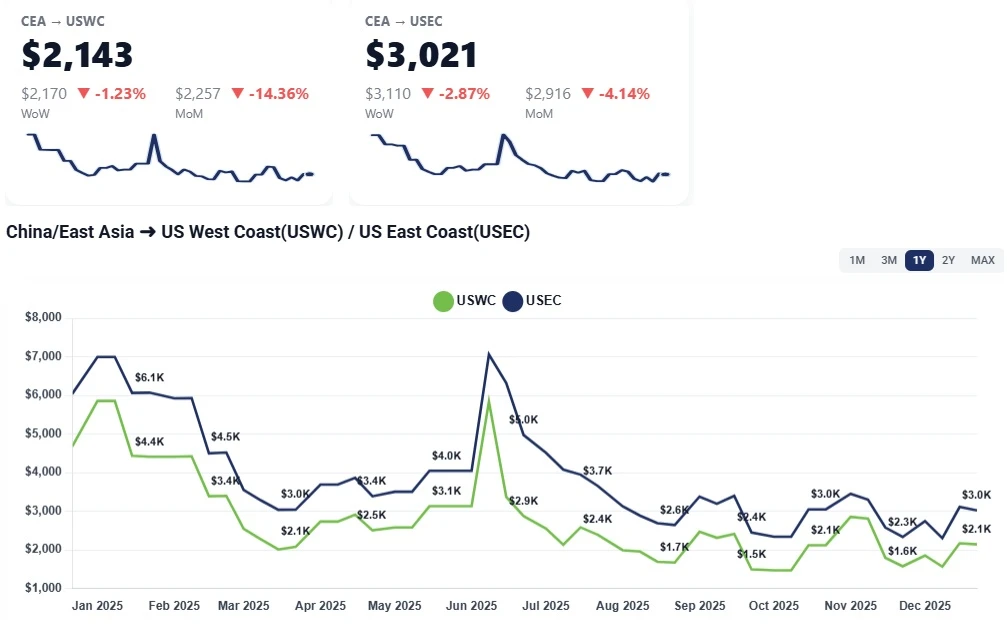
China–US ocean freight rates to the West and East Coasts held steady this week amid a holiday slowdown. Learn what’s driving the flat market and why January GRIs could push prices higher.

Founder & CEO of Freight Right Global Logistics, Robert Khachatryan, sat down with Tuck Ly, Vice President of Clearpoint International, to discuss the major issues affecting the global supply chain and port congestion

Demurrage and detention fees are mostly avoidable when you use the right solutions and partnerships to help you manage your shipments.

A list of strict ATA Carnet accepting-countries and the specifications they hold. Most non-commercial, exhibiting importers and exporters recognize the value and role of ATA Carnets in the complicated field of trade. While the World Customs Organization

President Trump issues Executive Order to stop overlapping tariffs on imports under national security and trade actions, ensuring duty rates remain targeted and non-cumulative. Effective retroactively from March 4, 2025.

The U.S. will impose 10% tariffs on lumber and 25% on furniture starting October 14, 2025, a move set to impact housing costs, supply chains, and trade relations.

FEU & TEU rates change slightly week-over-week, importers that can afford to keep importing are continuning to do so while those that are hamstrung by tariffs are sidelined and the August 1st tariff deadline is one week away.

The amount of truckers is dwindling and it is not good news for the freight industry. Why is this happening and what are the long and short term solutions?

Learn the top 2 reasons for eCommerce cart abandonment—high shipping costs and slow delivery—and discover actionable strategies to reduce lost sales and boost conversions.